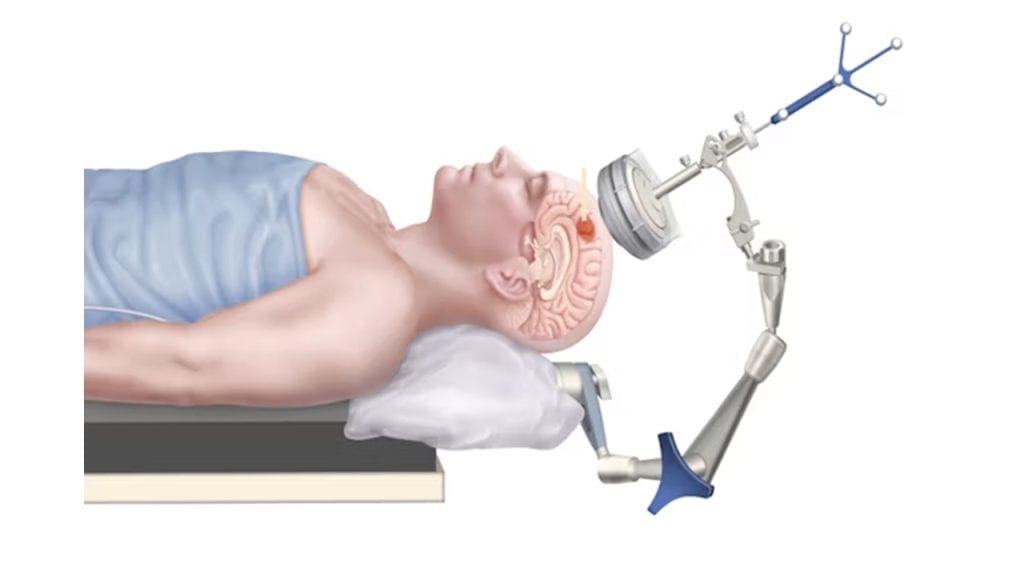
Dr. Hong Chen and her team have been utilizing focused ultrasound with microbubbles to open the blood-brain barrier for drug delivery or biomarker retrieval, but lacked a strategy to ensure the safety and consistency of their treatment. To address this, the team, which includes doctoral student Chih-Yen Chien, developed a quality assurance strategy that incorporates an acoustic sensor into their FUS device. The sensor passively captures acoustic signals to assess three quality assurance functions: ensuring consistent FUS device functionality, detecting air bubbles in the transducer via acoustic coupling, and evaluating the consistency of the treatment procedure. This quality assurance protocol was successfully tested in human trials, demonstrating its innovative approach to evaluate focused ultrasound systems for blood-brain barrier opening and potential other applications. Results of this study are published online in eBioMedicine.
Quality assurance protocol developed for focused ultrasound technique