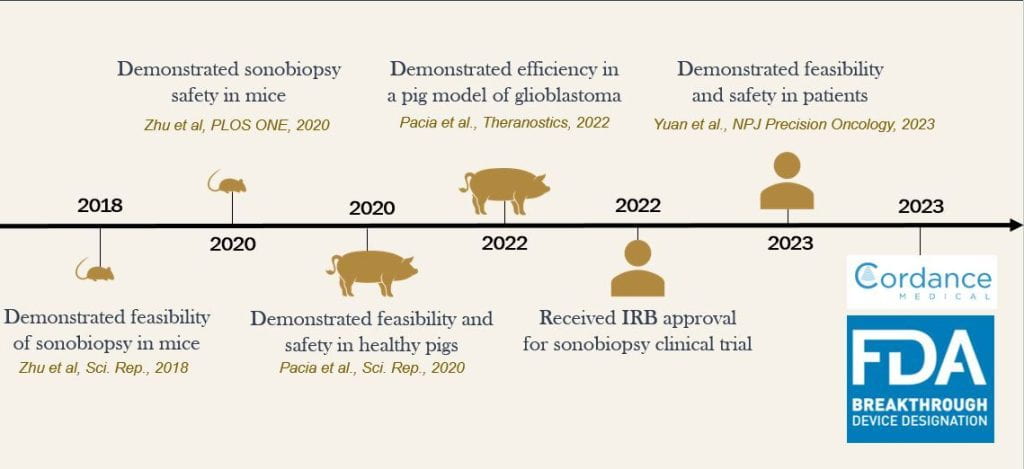
In just 5 years, our team turned the revolutionary Sonobiopsy technology from concept to commercialization amid the COVID-19 pandemic. This technology provides a noninvasive solution to brain disease diagnostics through the use of focused ultrasound for release of disease-specific biomarkers from brain to blood, which can further be detected and characterized using liquid-based biopsy. Cordance Medical has partnered with our team and has licensed key intellectual property for application of Sonobiopsy to their NeuroAccess device platform. We’re thrilled to announce that Cordance Medical’s NeuroAccess device has been granted FDA Breakthrough Device Designation for Sonobiopsy. More information on this recent development can be found here and here. Congratulations to the Chen team!
Cordance Receives FDA Breakthrough Device Designation for Sonobiopsy